Navigating the Evolving Landscape of Cannabis Usage in Clinical Trials
In the realm of clinical trials, cannabis has become a topic of intense debate and scrutiny. The constantly shifting legal status of cannabis adds layers of complexity, influencing patient recruitment, study conduct, and the future landscape of medical treatments for prevalent conditions. In this discussion, we will explore the intricate complexities, crucial considerations, and potential future paths for cannabis within the sphere of clinical research.
The Changing Legal Landscape
One of the major challenges with cannabis is its constantly shifting legal status. In the United States, the use and possession of cannabis is illegal under federal law for any purpose by way of the Controlled Substances Act of 1970 (CSA). However, the medical use of cannabis is legal with a medical recommendation in 38 states, four out of five permanently inhabited U.S. territories, and the federal District of Columbia (D.C.). The recreational use of cannabis has been legalized in 23 states, three U.S. territories, and D.C. Another eight states have decriminalized its use. Cannabis is fully illegal in four states: South Carolina, Idaho, Kansas, and Nebraska1.
Marijuana is currently classified as a Schedule I substance under the Controlled Substances Act (CSA) in the United States, which means it has “a high potential for abuse and no currently accepted medical use.” However, there have been recent developments that suggest a potential shift in marijuana’s legal status. In August 2023, the Department of Health and Human Services (HHS) Secretary Xavier Becerra announced that the department would recommend moving marijuana from Schedule I to Schedule III, which would mean that marijuana would be considered a substance with “moderate to low abuse potential, a currently accepted medical use, and a low potential for psychological dependence.” If marijuana is rescheduled to Schedule III, it would be much easier to research the drug in the United States2.
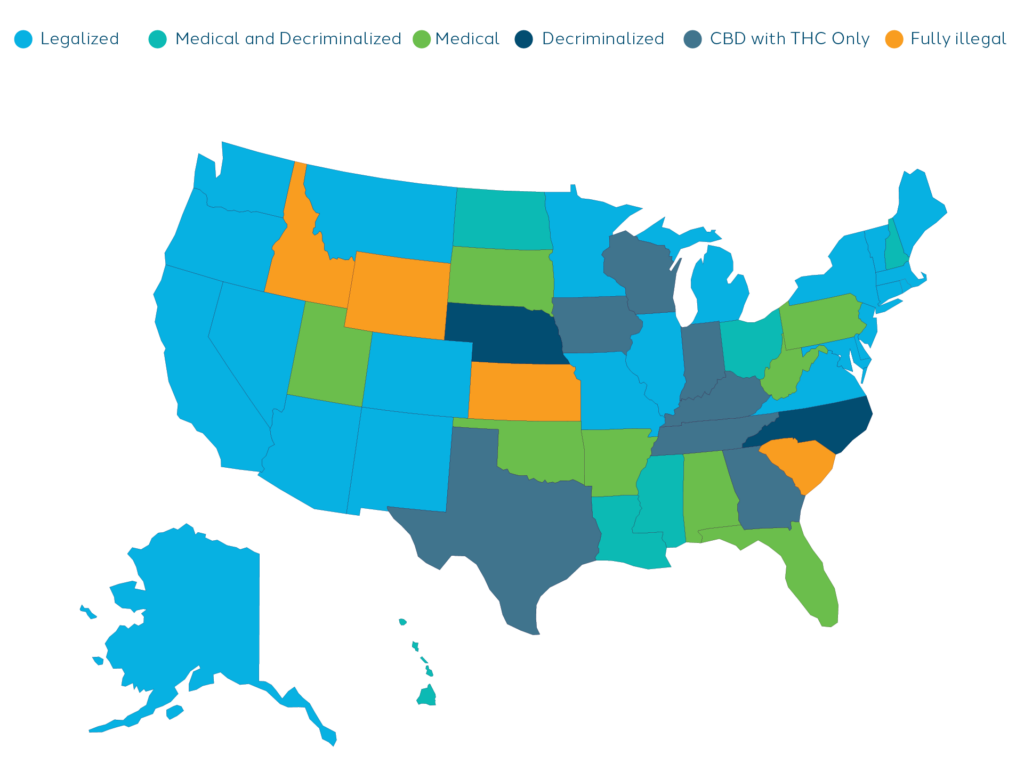
Understanding the nuances of local and federal laws is important as it directly influences patient recruitment, study protocols, and the subsequent interpretation of results.
The Impact on Patient Recruitment
Cannabis has also complicated recruitment of patients for clinical trials. The ambiguous language used in sponsors’ inclusion and exclusion criteria concerning cannabis poses one of the largest challenges in patient recruitment. Researchers are tasked with aligning the protocol’s wording with the sponsor’s interpretation and balancing medical judgment and legal regulations. Each study protocol uses varied language with attempts to make certain exceptions for cannabis usage. For example, one study permitted limited cannabis usage, allowing patients to engage in the prescreening phase or up to 6 months prior to the start of the study, but patients must stop once the study begins. Others require participants to undergo a urine drug screen and allow investigators to interpret results to be indicative of abuse or recreational use to determine participation in the study. However, this leads to wide variation of participant inclusion among investigators. There is a critical need for sponsors to specify cannabis usage in protocols, detailing both the quantity and relevance to study objectives.
Some indications being researched have been associated to higher rates of cannabis usage, making it more difficult to find suitable participants. Higher cannabis usage has been linked with certain conditions related to chronic pain, sleep disorders, and mental health and nervous system conditions, such as depression and anxiety3. While cannabis has been shown to be an effective treatment for some of these conditions, the FDA has not approved medical marijuana for treatment of depression as it has only been shown to alleviate depressive symptoms in the short term. The influence of marijuana on specific individuals, coupled with the implications of its usage based on participants’ co-diagnoses, has substantial effects on patient recruitment.
Pragmatic Study Designs: Adapting to the Reality of Cannabis
Flexible and pragmatic study designs are necessary to help incorporate cannabis as a variable in clinical research. Striking a balance between scientific rigor and real-world applicability is essential, especially given the prevalence of cannabis usage.
Maintaining the scientific integrity of the clinical study is essential. Investigators must maintain pristine study conditions, devoid of other substances, to ensure the accuracy of research outcomes. And cannabis possesses psychoactive properties that might influence the outcomes of clinical trials. Having sponsors clearly delineate acceptable levels of cannabis usage for study participation would help with this, but clinical trials must also adapt to the shifting legal landscape and the implications of recreational cannabis use.
As more and more states take steps to legalize marijuana for medical and recreational use, embracing pragmatism is equally as vital. The rise of dispensaries offering cannabis in diverse forms, from tinctures to edibles and beverages, is causing the amount of cannabis users to skyrocket and necessitates the inclusion of cannabis users in clinical trials. It’s also important for investigators to understand how cannabis impacts the efficacy of the drug being studied.
By focusing on pragmatic study designs, researchers can gain valuable insights into how cannabis operates across diverse individuals and conditions, ensuring a comprehensive and nuanced understanding of its effects in a real-world context. One approach could involve forming study groups comprising of cannabis users, allowing for comparisons against non-cannabis user groups and controls. Additionally, monitoring patients post-study, especially when they continue the medication alongside cannabis use, provides a comprehensive view of the drug’s effectiveness amidst real-life cannabis consumption. This pragmatic perspective ensures that clinical research remains relevant and reflective of the evolving cannabis landscape.
The Role of Medical Marijuana
The medicinal promise of cannabis cannot be dismissed. Cannabis has shown significant potential in alleviating symptoms associated with anxiety, sleep disorders, chronic pain, and more. Lowering cannabis from a Schedule I to a Schedule III substance can open the doors to more research studying the various benefits cannabis has on commonly studied indications.
One of the most prevalent uses in the United States is for pain relief, where marijuana demonstrates effectiveness in alleviating multiple sclerosis-related pain, nerve pain, and chronic pain, particularly neuropathic pain4. Additionally, studies have shown the positive impact of CBD, a non-psychoactive component in cannabis, in treating epilepsy and reducing symptoms and seizure frequency in various seizure syndromes5. Another noteworthy area is the treatment of gastrointestinal (GI) disorders, where non-psychoactive cannabinoids like CBD prove effective in managing conditions such as irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), Crohn’s, and ulcerative colitis6. Cannabis also plays a role in appetite stimulation, aiding individuals with cancer and AIDS by improving weight gain7. Notably, its potential in helping veterans with PTSD has sparked significant interest, with numerous reports indicating substantial improvement and a strong demand for more research in this area.
While marijuana offers potential relief for certain medical conditions, it’s crucial to acknowledge the limitations. The FDA has not universally approved medical marijuana for treatment. Additionally, it’s essential to recognize the negative impact of cannabis use on mental health in some patient profiles. Cannabis’s complex profile demands careful consideration, emphasizing the need for informed medical guidance to ensure its safe and appropriate use as a treatment option. As cannabis becomes increasingly prevalent in society, its evolution into a medical treatment further complicates clinical research, demanding innovative approaches to study its impact.
Looking Towards the Future
Investigators and sponsors need to acknowledge that cannabis is here to stay and research methodologies need to adapt to this new dynamic landscape, marked by shifting legalities and the growing acceptance of cannabis as medical treatment. Pragmatic study designs, balancing scientific rigor with real-world applicability, offer a promising approach, allowing researchers to navigate cannabis’s impact across diverse individuals and conditions. By embracing these principles, the medical community can unravel the potential of cannabis while ensuring ethical, comprehensive, and impactful clinical trials.
Webinar – Green Frontiers: Cannabis Impact On Patient Recruitment & Outcomes
To enhance your understanding, watch our webinar recording with SCRS on cannabis usage in clinical trials. Explore the complex world of cannabis use in the U.S. and its impact on patient recruitment and clinical trial outcomes. Site leaders and investigators will guide discussions on current trends, legal aspects, and the physiological/psychological effects on trial participants. Learn protocol strategies and best practices for recruiting participants using cannabis while optimizing your approach to cannabis-related considerations in clinical research. Access the recording here.
References
- State medical cannabis laws. (2023, October 2). https://www.ncsl.org/health/state-medical-cannabis-laws
- Simmons-Duffin, S. (2023, August 31). Marijuana could soon be downgraded from a Schedule 1 drug. NPR. https://www.npr.org/2023/08/31/1197084320/marijuana-could-soon-be-downgraded-from-a-schedule-1-drug
- Black N, Stockings E, Campbell G, Tran LT, Zagic D, Hall WD, Farrell M, Degenhardt L. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2019 Dec;6(12):995-1010. doi: 10.1016/S2215-0366(19)30401-8. Epub 2019 Oct 28. Erratum in: Lancet Psychiatry. 2020 Jan;7(1):e3. PMID: 31672337; PMCID: PMC6949116.
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Health Effects of Marijuana: An Evidence Review and Research Agenda. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research. Washington (DC): National Academies Press (US); 2017 Jan 12. 4, Therapeutic Effects of Cannabis and Cannabinoids. Available from: https://www.ncbi.nlm.nih.gov/books/NBK425767/
- Rosenberg, E.C., Tsien, R.W., Whalley, B.J. et al. Cannabinoids and Epilepsy. Neurotherapeutics 12, 747–768 (2015). https://doi.org/10.1007/s13311-015-0375-5
- Ahmed W et al. Therapeutic Use of Cannabis in Inflammatory Bowel Disease. Gastroenterology and Hepatology. 2016;12(11):668-679
- Ostadhadi S et al. Therapeutic potential of cannabinoids in counteracting chemotherapy-induced adverse effects: an exploratory review. Phytotherapy Research. 2015;29(3):332-8




